binax now covid test too many drops|We reviewed three at : discount store The BinaxNOW COVID-19 Antigen Self-Test is a rapid test that uses a shallow nasal swab sample to check for the presence or absence of proteins from the virus that causes COVID-19. Here’s. web30 anos. Cabo Frio RJ. Uma acompanhante com estilo doce agradável e encantador, ideal para quem busca fugir da rotina de uma forma intimista e atenciosa. Além de discreta .
{plog:ftitle_list}
Welcome to the world of Kaboo! Start your Casino adventure .
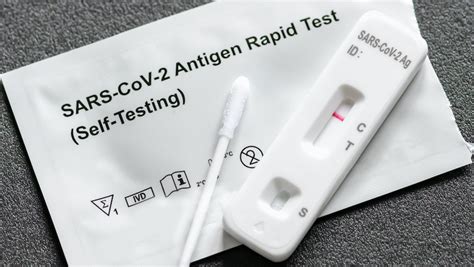
The BinaxNOW™ COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. This test is authorized for non-prescription home . I had to drop some reagent liquid in the correct hole on the test card. The instructions said to hold the bottle straight, not at an angle, and to make sure to get six drops in. The BinaxNOW COVID-19 Ag Card Home Test is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid .The BinaxNOW COVID-19 Antigen Self Test is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from direct .
The BinaxNOW COVID-19 Antigen Self-Test is a rapid test that uses a shallow nasal swab sample to check for the presence or absence of proteins from the virus that causes COVID-19. Here’s.The BinaxNOW TM COVID-19 Ag Card is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from nasal swab .
The three tests we tried included two antigen tests, BinaxNow from Abbott Laboratories and a kit from Ellume, as well as one molecular test, called Lucira. The paper, which has not been peer-reviewed, found that two common rapid tests, Abbott’s BinaxNOW and Quidel’s QuickVue, often failed to detect infection for a few days after .

BinaxNOW COVID-19 Antigen Self Test. Extended Expiration Date; People with symptoms that began within the last 7 days. The test is to be performed two times over three days (serial .Testing has been completed to support a shelf-life (expiration date) of up to 22 months. The BinaxNOW™ COVID-19 Antigen Self Test, part number 195-160 or 195- 180, may now have a longer than labeled product expiry date. All . The BinaxNOW COVID-19 Antigen Self-Test is an FDA-approved at-home test kit. It can detect active infections with SARS-CoV-2, the virus that causes COVID-19, in people with and without symptoms.
Contents: 2 BinaxNOW Covid-19 test cards; 2 nasal swabs; 2 reagent bottles. binaxnow-selftest.abbott. For use under emergency. Use authorization only. Store between 35.6-86 degrees F (2-30 degrees C) until use. ©2021 Abbott.The BinaxNOW COVID-19 Antigen Self Test is a rapid lateral flow immunoassay for the qualitative detection of SARS-CoV-2 directly from anterior nasal swabs, without viral transport media. The BinaxNOW COVID-19 Antigen Self Test kit contains all components required to carry out an assay for SARS-CoV-2. PRINCIPLES of the PROCEDUREBinaxNOW COVID-19 Antigen Self Test. Extended Expiration Date; People with symptoms that began within the last 7 days. The test is to be performed two times over three days (serial testing).
Molecular tests that run on our m2000 system have the ability to run high volumes of up to 470 tests in 24 hours.Learn more about m2000 here: https://abbo.tt/3b8bASF Alinity m systems have the ability to run high volumes of up to 1,080 tests in 24 hours. Learn more about Alinity m here: https://abbo.tt/2zrt52N ID NOW delivers positive results in as little as 5 minutes .Patient Samples require 6 drops of Extraction Reagent. Wrong . 1 . . Individuals who test positive with the BinaxNOW COVID-19 Ag Card should self-isolate and seek follow up care with their

According to the Department of Health & Human Services, beginning January 15, 2022, individuals with private health insurance coverage or covered by a group health plan who purchase an over-the-counter COVID-19 diagnostic test authorized, cleared, or approved by the U.S. Food and Drug Administration (FDA) will be able to have those test costs covered by .
Accuracy issues. The issue with home tests is accuracy, which is between 85% and 95% for detecting covid. That is, they catch about nine of every 10 infections, a metric called the test’s . Which is why we're sharing the latest updates with our BinaxNOW COVID-19 Self Tests so they can continue to have a positive impact in your lives even as COVID-19 and its many variants — including the XBB.1.5 or "Kraken" you may have recently heard about — continue to change. Update 1: Your BinaxNOW Self Test May Have a Longer Shelf Life JN.1, the now-dominant COVID-19 variant that accounts for nearly 86% of all currently circulating SARS-CoV-2 strains, may take longer to show a positive result on home antigen tests.
We reviewed three at
The Abbott BinaxNOW, a widely used at-home coronavirus test, can detect most people who are infected with the new Omicron variant and are carrying high levels of the virus, according to a new .
www.fda.gov . Individuals using assistive technology may not be able to fully access the information contained in this document. For . assistance, please call 1(800) 638-2041 or (301) 796-7100 orOn Friday (March 18), the FDA issued an alert warning that at-home COVID-19 tests can cause harm if they are used improperly, for example, if the liquid test solution touches a person's skin or .The BinaxNOW COVID -19 Ag Card 2 Home Test is intended for observed non-prescription self - use and/or, as applicable for an adult lay user testing another person aged 2 years or older in a non .
COVID-19 test may be necessary, depending on your individual risk factors and test results. Your sample(s) was tested for COVID-19 using the BinaxNOW™ COVID-19 Ag Card. This Fact Sheet contains information to help you understand the risks and benefits of using this test for the diagnosis of COVID-19.
The BinaxNOW COVID-19 Antigen Self Test is a rapid lateral flow immunoassay for the qualitative detection of SARS-CoV-2 directly from anterior nasal swabs, without viral transport media. The BinaxNOW COVID-19 Antigen Self Test kit contains all components required to carry out an assay for SARS-CoV-2. PRINCIPLES of the PROCEDURE*Be sure there is a blue line present at the Control Line prior to running the test (discard the test card if there is no blue line). Step 1: Add the extraction reagent to the BinaxNOW COVID-19 Ag Card by holding the bottle vertically (NOT at an angle), hovering ½ inch above the TOP HOLE, slowly adding eight (8) drops.To perform the test, a nasal swab specimen is collected from the patient, 6 drops of extraction reagent from a dropper bottle are added to the top hole of the swab . Nasal Swabs (40): Sterile swabs for use with BinaxNOW COVID-19 Ag Card test Positive Control Swab (1): Non-infectious recombinant SARS-CoV-2 nucleocapsid antigen dried onto a swab A positive COVID-19 test means the virus was detected and you have or recently had an infection. Take steps to prevent spreading COVID-19. Monitor your symptoms. If you have any emergency warning signs, seek emergency care immediately. Seek health care right away for treatment if you have risk factors for severe illness. Treatment may be an .
The BinaxNOW™ COVID-19 Antigen Self Test has been designed to minimize the likelihood of false positive test results. However, in the event of a false positive result, risks could include theAbbott BinaxNOWTM COVID-19 Ag Card Test Helpful Testing Tips (continued) Before the Test . 1 . Store kit at 2-30°C (35.6-86° F). Do not freeze kits. Test kit reagents/cards must be at room temperature before use. If stored in a refrigerator, allow time to warm up to room temperature. Only the swabs provided with the kit are approved for use .
The BinaxNow, BD Veritor, Flowflex, and Celltrion DiaTrust COVID-19 rapid antigen kits all contain this chemical. Sodium azide is a colorless, tasteless, and odorless powder that has been used as a propellant in automobile airbags, an herbicide, and a pest control agent. . Case #2: An adult woman mistook the test kit vial from her home COVID .
A COVID-19 rapid lateral flow test shows you the result on a device that comes with the test. How to do a COVID-19 rapid lateral flow test. How you do the test depends on the test kit you’re using. Rapid lateral flow tests require either a: throat and nose swab; nose swab only; The test you have might be different to one you've done before so .A simple solution for COVID-19 infection detection, with rapid results in the convenience of your home. This test has received FDA Emergency Use Authorization for self-testing without the need to ship samples to a lab or for a prescription from your healthcare provider. This 15-minute test can be completed anytime, anywhere. Serial testing should be performed in individuals with .
Q&A on At
The BinaxNOW™ COVID-19 Ag Card provides COVID-19 test results quickly and easily. This simple, CLIA-waived test provides visual results in just 15 minutes. CONTACT. DIAGNOSTICS. ABOUT ABBOTT. . The BinaxNOW COVID-19 Ag Card test has received U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA).* .
She’s a Rich Girl Elena Pérez Elena lleva desde el año 2013 colaborando con distintos medios nacionales e internacionales relacionados con el ámbito de las apuestas en línea, los juegos de casino y el sector eGaming en su conjunto.
binax now covid test too many drops|We reviewed three at